SAP Validation plays a crucial role across many industries. However, when it comes to pharma and life science, the significance increases multifold as these companies are under a lot more scrutiny from the government agencies. The reason is quite obvious as these businesses deal with the entire development, production, and supply of pharmaceutical products.
SAP Validation in Pharma: Why It is Important
The pharmaceutical products are quite sensitive as they can be a boon or a bane. Thus, for the purpose of preventing diseases and saving lives, these products should be as safe and effective. They shouldn’t pose significant danger to life by means of any potential side-effects. That’s why SAP validation in pharma should not be taken lightly by any means.
Let’s see three primary reasons why SAP validation in
- Ensure Product Quality: Pharmaceutical companies produce medicines, drugs and equipment that hospitals use worldwide to treat patients. This means that the manufacturing process must be extremely safe, clean, and top-quality.
- Compliance with Government Policies: The government forms various policies for pharmaceutical companies which include guidelines, rules, and procedures. Teams from government agencies regularly go on surprise visits to inspect the companies. They want to make sure that all regulations are being followed. A company could be shut down if it’s found violating the norms.
The agencies that administer implementation of these policies are Drug Controller General of India (DCGI) in India, Food & Drug Administration (FDA) in the US and European Medicines Evaluation Agency (EMEA) in Europe.
- Perform Timely Upgrades: Pharma is such an industry that witnesses rapid changes on a consistent basis. It includes government regulations, product development processes, technology, etc. Thus, the pharma companies need to adopt these regulations and processes by evaluating, studying and modifying them on a regular basis. SAP Validation in pharma helps you stay ahead of these changes.
System Validation in Pharma: V- Model Concept
Below is the universally accepted model, commonly known as V model. The left side of the V deals with defining and detailing the change. Meanwhile, the right side of the V ensures that for every item in the left hand, there is a corresponding activity to verify that the change has been made as per the design and requirements. Each Pharma company, IT Department and Implementation partner (System Integrator), has its own Quality System. (both validation and verification process.
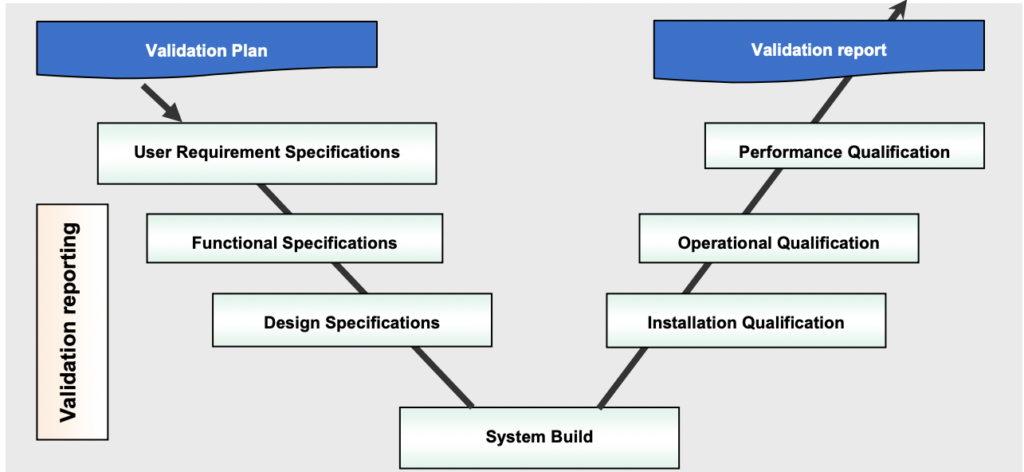
Here’s what each step represents:
User Requirement Specifications: This step determines the requirements of the user and ensures that they are satisfied
Functional Specifications: This step defines and creates a document that is user-friendly based on URS. This is done by a vendor or developer.
Design Qualification: This step is a document that clearly defines the requirements for computer systems and is in line with the user requirement specifications. It also includes a document that outlines all system specifications, which have been reviewed and approved.
Installation Qualification: This step document will ensure that all aspects of hardware installation have been considered in accordance with approved codes and designs. Software installation is also covered in this step.
Operation Qualification: This step makes sure that the overall system works as intended and there are no loose ends.
Performance Qualification: This step provides the guarantee that all components of the system work as expected within all operating conditions.
SAP CSV Validation: What it Includes
SAP, in its document, suggests validation of all computer systems used for or performing regulated operations. Here’s what SAP CSV validation includes:
- System used to control the quality of regulated products during various life cycle stages of the product (development, testing, manufacture, etc).
- Systems that create, modify, store, transmit regulated data such as product safety data, clinical trial data, product efficacy data, etc.
- Systems that maintain decision making data.
- Systems used to maintain data to be made available for agency inspection.
- Systems used to submit electronic records to the agency.
Sondagar: Your SAP/ERP Validation Partner
We, at Sondagar, are proud to partner with many companies in the life sciences industry. We have worked with top Biotech, Pharmaceutical and Medical Device companies to implement and develop their SAP systems. Our specialization includes SAP/ERP validation protocols like Microsoft Dynamics, SAP and Oracle (JREdwards & e-business Suite).
Our years of experience have helped us create SAP validation processes which are cost-effective, flexible, and risk-based. We offer SAP validation services that cover the entire lifecycle of SAP validation, including implementation, decommissioning, and data archiving. It includes templates, procedures, training materials, and tools for software verification.
Schedule a free consultation to know how we can help you!

